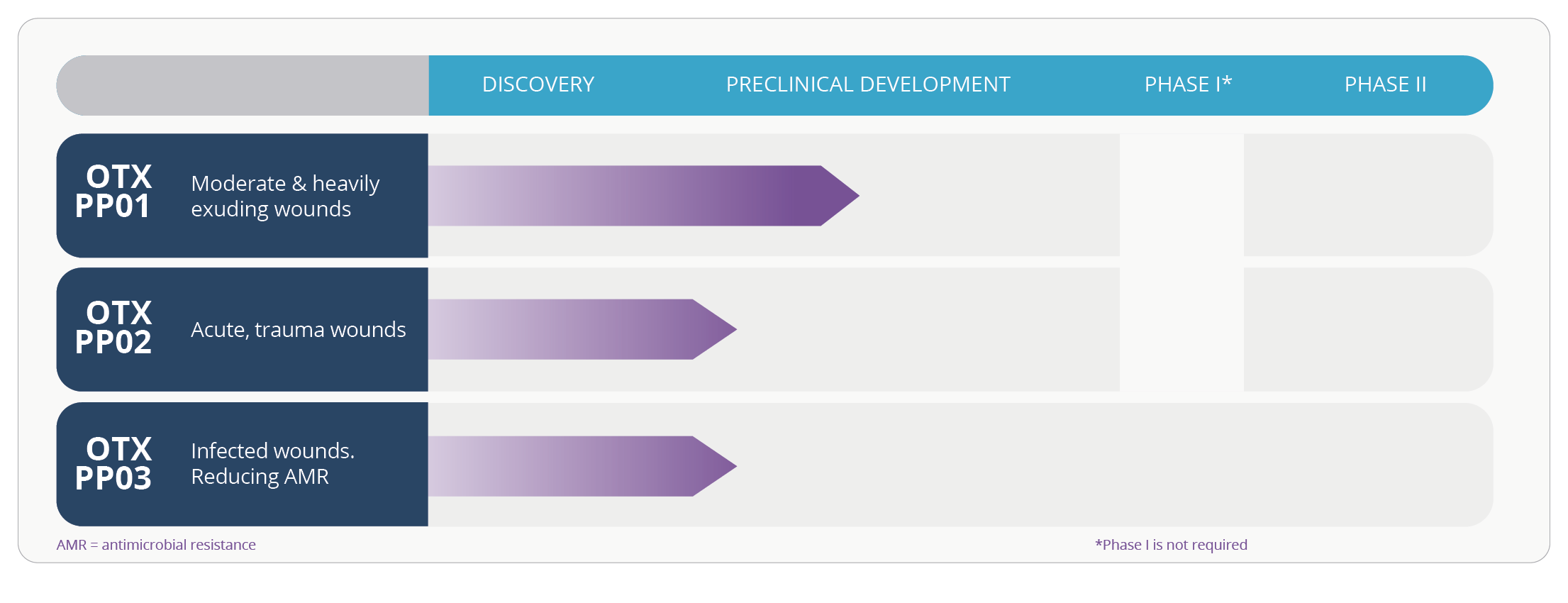
OTX PP01 & OTX PP02 are de-risked projects with a fast time to market. Due to the historical usage of the active compound and the clinical safety and toxicity data available, there is no requirement for a Phase I clinical study.
OTX PP01 is a controlled drug delivery patch treating excess exudate. This results in quicker healing times while improving patients quality of life through less frequent dressing changes and complications associated with excess exudate and/or infection. This will reduce the clinical and financial burden of expensive wound management tools, while enabling wounds to return to their healing state.
OTX PP02 is being designed for acute wounds at high risk of infection. The key advantages of OTX PP02 is a targeted and sustained release rate and extended application time.
Our Capabilities
Onya’s diverse and experienced team has a proven track record of delivering innovative, market leading products globally.
- Our external healthcare experts have sector specific knowledge across pharmaceutical, medical device and biotech industries. Bolstering resources in pre-clinical, product development, quality, engineering, regulatory, clinical and commercial.
- We have established a clinical advisory board of wound care specialists as part of the clinical trials programme.
- Advisory Board; Internationally recognised podiatrists, diabetologists, nurse specialists in tissue viability, skin integrity, infection and pressure ulcers, vascular surgeons, burns and plastics surgeons

